

- #Fe periodic table chemistry full
- #Fe periodic table chemistry plus
- #Fe periodic table chemistry series
#Fe periodic table chemistry full
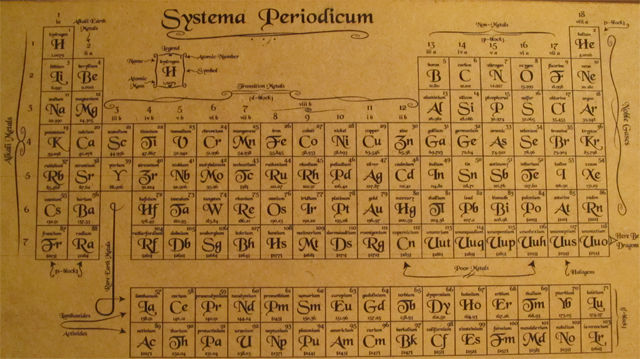
As expected, ions with no d-orbital electrons, like Sc +3, or a full d orbital, like Zn +2, are colorless. Depending on the energy difference between those d orbitals, different wavelengths of light will be absorbed and reflected. For example, Cu +2 is colorless, but Cu(H 20) 6 +2 is a blue color. But when a transition metal forms a complex with a ligand, such as H 2O or NH 3, the d orbitals develop different energy levels. The d orbitals are normally degenerate, meaning they are all at the same energy level. When a transition metal forms an ion, its electrons can absorb light and move between d orbitals. It is because of their unfilled d orbitals, and something called “d to d electronic transitions”. So you end up with interesting compounds like ferric ferrocyanide Fe 4 3, aka prussian blue, or copper (I) tetraiodomercurate, Cu 2HgI 4. Many of these metals form complexes, called coordination compounds, with ligands ranging from H 20 or halides to organic molecules such as EDTA. For example, manganese can easily be put into 5 different oxidation states. In their elemental form, they often act as catalysts.Īlmost all exhibit multiple oxidation states, especially the metals in groups 5,6,7, and 8. They often form paramagnetic compounds because of their unpaired d electrons. Transition metal compounds are often highly colored, due to d to d electron transitions. Some of the most common examples include iron, chromium, manganese, vanadium, titanium, copper, cobalt, nickel, tungsten, gold, and platinum. Transition metal ions usually have incompletely filled d orbitals, except for scandium – which is why scandium is sometimes excluded as a transition metal. When forming anions, these metals always lose their s-orbital electrons first.


They are often good catalysts, and readily form complexes with molecules called ligands. They are usually quite dense, and are less reactive than a alkali or alkaline earth elements. They are hard solids, with high melting points and boiling points. Their compounds are often brightly colored in solution and when hydrated, and can exhibit multiple positive oxidation states. Transition metals are generally good conductors of heat and electricity, malleable and ductile. The English chemist Charles Bury first used this term, to describe this group of elements. It is sometimes used interchangeably with the term transition metal. “D block” elements is a commonly used phrase, that describes the elements in groups 3 to 12, They are called d-block elements because as you go across the rows, the d-orbital gradually gets filled with electrons. This definition excludes scandium, since the Sc+3 ion does not have unpaired d electrons. Unpaired d electrons are more likely to participate in chemical reactions. One definition of a transition metal, is any metal that has at least one unpaired d electron in one of their stable ions. There is no one universally agreed definition of what a transition metal is.
#Fe periodic table chemistry series
Not included in this definition are the lanthanides or the actinide series elements, often called “inner transition metals”, or zinc, cadmium or mercury, which we call “post-transition metals”.
#Fe periodic table chemistry plus
In this article, we will consider the transition metals, aka transition elements, to includes the elements of the periodic table from groups 4 to 11, plus scandium and yttrium. But what is a transition metal? There are several different definitions. When most of us think of “metals”, we are probably thinking of a transition metal.


 0 kommentar(er)
0 kommentar(er)
